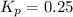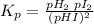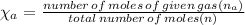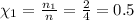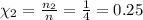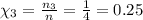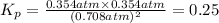Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1



Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2
You know the right answer?
Areaction vessel is charged with hydrogen iodide, which partially decomposes to molecular hydrogen a...
Questions


Mathematics, 25.04.2020 05:02










Social Studies, 25.04.2020 05:02


Mathematics, 25.04.2020 05:02




Computers and Technology, 25.04.2020 05:02