
An equilibrium mixture of pcl 5 ( g ) , pcl 3 ( g ) , and cl 2 ( g ) has partial pressures of 217.0 torr, 13.2 torr, and 13.2 torr, respectively. a quantity of cl 2 ( g ) is injected into the mixture, and the total pressure jumps to 263.0 torr. the appropriate chemical equation is pcl 3 ( g ) + cl 2 ( g ) − ⇀ ↽ − pcl 5 ( g ) calculate the new partial pressures after equilibrium is reestablished.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2
You know the right answer?
An equilibrium mixture of pcl 5 ( g ) , pcl 3 ( g ) , and cl 2 ( g ) has partial pressures of 217.0...
Questions


Social Studies, 23.04.2020 20:21

Chemistry, 23.04.2020 20:21

Mathematics, 23.04.2020 20:21

English, 23.04.2020 20:21






Mathematics, 23.04.2020 20:22

Mathematics, 23.04.2020 20:22


History, 23.04.2020 20:22






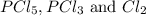 when equilibrium is re-established are 223.4 torr, 6.82 torr and 26.4 torr respectively.
when equilibrium is re-established are 223.4 torr, 6.82 torr and 26.4 torr respectively.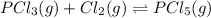
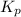 for above reaction follows:
for above reaction follows: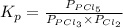 ........(1)
........(1)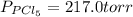
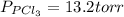
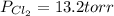
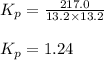
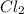 .
.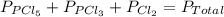
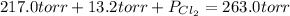
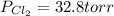
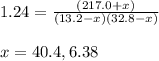
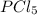 = (217.0+x) = (217.0+6.38) = 223.4 torr
= (217.0+x) = (217.0+6.38) = 223.4 torr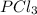 = (13.2-x) = (13.2-6.38) = 6.82 torr
= (13.2-x) = (13.2-6.38) = 6.82 torr

