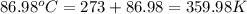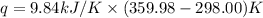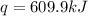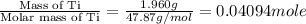Chemistry, 11.12.2019 23:31 cheyennemitchel2680
When 1.960 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00 °c to 86.98 °c. in a separate experiment, the heat capacity of the calorimeter is measured to be 9.84 kj/k. the heat of reaction for the combustion of a mole of ti in this calorimeter is kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
When 1.960 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter incr...
Questions


Mathematics, 12.09.2021 04:50

English, 12.09.2021 04:50


Mathematics, 12.09.2021 04:50



Advanced Placement (AP), 12.09.2021 04:50

Spanish, 12.09.2021 04:50


Mathematics, 12.09.2021 04:50


Mathematics, 12.09.2021 04:50


Mathematics, 12.09.2021 04:50

English, 12.09.2021 04:50


Arts, 12.09.2021 04:50


Mathematics, 12.09.2021 04:50

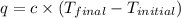
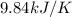
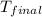 = final temperature =
= final temperature = 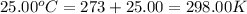
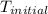 = initial temperature =
= initial temperature =