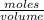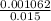Chemistry, 11.12.2019 23:31 asndjsd9397
Asolution of sodium hydroxide, 0.0500 m, is used to titrate a 15.00 ml sample of hydrochloric acid to the endpoint. the initial buret reading was 3.87 m; the final reading was 25.11 ml. what is the molarity of the acid solution.?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2
You know the right answer?
Asolution of sodium hydroxide, 0.0500 m, is used to titrate a 15.00 ml sample of hydrochloric acid t...
Questions

Mathematics, 02.07.2020 01:01


Mathematics, 02.07.2020 01:01

Mathematics, 02.07.2020 01:01


English, 02.07.2020 01:01


Mathematics, 02.07.2020 01:01


Mathematics, 02.07.2020 01:01

Mathematics, 02.07.2020 01:01


Mathematics, 02.07.2020 01:01





Mathematics, 02.07.2020 01:01