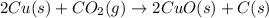The reaction 2cu(s) + co2(g) → 2cuo(s) + c(s) is endothermic. which of the following statements is true?
a) the reaction is not spontaneous at any temperature.
b) the reaction is spontaneous at all temperatures.
c) it is impossible to determine if the reaction is spontaneous without calculations.
d) the reaction will be spontaneous only at low temperatures.
e) the reaction will be spontaneous only at high temperatures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
The reaction 2cu(s) + co2(g) → 2cuo(s) + c(s) is endothermic. which of the following statements is t...
Questions

English, 23.09.2021 01:00

Mathematics, 23.09.2021 01:00





Mathematics, 23.09.2021 01:00






Social Studies, 23.09.2021 01:00

Mathematics, 23.09.2021 01:00



Social Studies, 23.09.2021 01:00


History, 23.09.2021 01:00

Mathematics, 23.09.2021 01:00