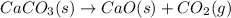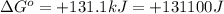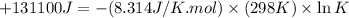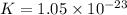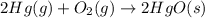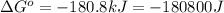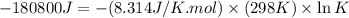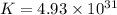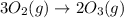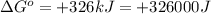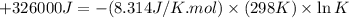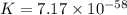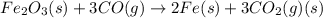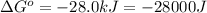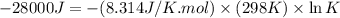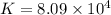Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
Which of the following reactions will have the largest value of k at 298 k? a) caco3(s) → cao(s) +...
Questions


Mathematics, 13.06.2021 22:40

Mathematics, 13.06.2021 22:40

Mathematics, 13.06.2021 22:40



Social Studies, 13.06.2021 22:40



English, 13.06.2021 22:40

Biology, 13.06.2021 22:40


Health, 13.06.2021 22:40



History, 13.06.2021 22:40

English, 13.06.2021 22:40



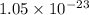
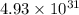
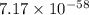
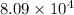
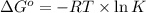
 = standard Gibbs free energy
= standard Gibbs free energy