
Chemistry, 11.12.2019 21:31 keniaguevara32
Calculate δhrxn for the following reaction: ch4(g)+2cl2(g)→ch2cl2(g)+2hcl(g) use the following reactions and given δh values. ch4(g)+cl2(g)→ch3cl(g)+hcl(g), δh=−99.60 kj ch3cl(g)+cl2(g)→ch2cl2(g)+hcl(g), δh=−105.8 kj express your answer to four significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1

You know the right answer?
Calculate δhrxn for the following reaction: ch4(g)+2cl2(g)→ch2cl2(g)+2hcl(g) use the following reac...
Questions

Mathematics, 05.06.2021 14:40


Health, 05.06.2021 14:40


Health, 05.06.2021 14:40

Law, 05.06.2021 14:40


Mathematics, 05.06.2021 14:40

Mathematics, 05.06.2021 14:40

Mathematics, 05.06.2021 14:50

Geography, 05.06.2021 14:50

Mathematics, 05.06.2021 14:50


English, 05.06.2021 14:50

Mathematics, 05.06.2021 14:50

Mathematics, 05.06.2021 14:50

Mathematics, 05.06.2021 14:50



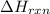 for the reaction is -205.4 kJ.
for the reaction is -205.4 kJ.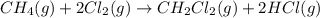
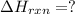
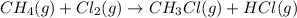
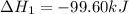
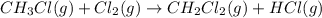
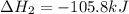
![\Delta H_{rxn}=[1\times \Delta H_1]+[1\times \Delta H_2]](/tpl/images/0414/0614/6e774.png)
![\Delta H_{rxn}=[(1\times (-99.60))+(1\times (-105.8))]=-205.4kJ](/tpl/images/0414/0614/3a575.png)


