
Chemistry, 11.12.2019 20:31 brainist71
Consider the following equilibrium at 970 k for the dissociation of molecular iodine into atoms of iodine. i2(g) equilibrium reaction arrow 2 i(g); kc = 1.35 ✕ 10−3 suppose this reaction is initiated in a 3.7 l container with 0.063 mol i2 at 970 k. calculate the concentrations of i2 and i at equilibrium.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

You know the right answer?
Consider the following equilibrium at 970 k for the dissociation of molecular iodine into atoms of i...
Questions

Chemistry, 18.07.2019 20:00


Physics, 18.07.2019 20:00

Chemistry, 18.07.2019 20:00


Business, 18.07.2019 20:00

Biology, 18.07.2019 20:00

Mathematics, 18.07.2019 20:00






Biology, 18.07.2019 20:00



History, 18.07.2019 20:00



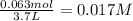
![Kc=1.35 \times 10^{-3} =\frac{[I]^{2} }{[I_{2}]} =\frac{(2x)^{2} }{(0.017-x)} \\4x^{2} +1.35 \times 10^{-3}x - 2.3 \times 10^{-5}](/tpl/images/0413/9700/35688.png)


