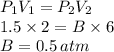Chemistry, 11.12.2019 19:31 preservations
How well can you apply boyle’s law to this sample of gas that experiences changes in pressure and volume? assume that temperature and number of moles of gas are constant in this problem.
using the first volume and pressure reading on the table as v1 and p1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
How well can you apply boyle’s law to this sample of gas that experiences changes in pressure and vo...
Questions



Mathematics, 27.04.2021 05:10

Computers and Technology, 27.04.2021 05:10

History, 27.04.2021 05:10

Social Studies, 27.04.2021 05:10



Chemistry, 27.04.2021 05:10

Mathematics, 27.04.2021 05:10

Mathematics, 27.04.2021 05:10

History, 27.04.2021 05:10

Geography, 27.04.2021 05:10



Mathematics, 27.04.2021 05:10



Mathematics, 27.04.2021 05:10