Chemistry, 11.12.2019 19:31 mariahrpoulin1511
The value of the solubility product constant for ag2co3 is 8.5 × 10‒12 and that of ag2cro4 is 1.1 × 10‒12. from this data, what is the value of kc for the reaction, ag2co3(s) + cro42‒(aq) → ag2cro4(s) + co32‒(aq) a) 9.6 × 10‒12 b) 7.7 c) 1.1 × 1023 d) 1.3 × 10‒1 e) 9.4 × 10‒24

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1


Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
The value of the solubility product constant for ag2co3 is 8.5 × 10‒12 and that of ag2cro4 is 1.1 ×...
Questions


Biology, 24.06.2019 15:30

Biology, 24.06.2019 15:30


Biology, 24.06.2019 15:30

Mathematics, 24.06.2019 15:30

Mathematics, 24.06.2019 15:30

English, 24.06.2019 15:30


Mathematics, 24.06.2019 15:30





Mathematics, 24.06.2019 15:30


Biology, 24.06.2019 15:30

English, 24.06.2019 15:30

Mathematics, 24.06.2019 15:30

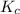 for the given reaction is 7.7
for the given reaction is 7.7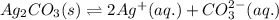
![K_{sp}(Ag_{2}CO_{3})=[Ag^{+}]^{2}[CO_{3}^{2-}]](/tpl/images/0413/8617/b8832.png)
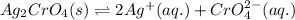
![K_{sp}(Ag_{2}CrO_{4})=[Ag^{+}]^{2}[CrO_{4}^{2-}]](/tpl/images/0413/8617/31f53.png)
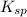 represents solubility product
represents solubility product![K_{c}=\frac{[CO_{3}^{2-}]}{[CrO_{4}^{2-}]}](/tpl/images/0413/8617/fea9e.png) (concentration of pure solids remain constant during reaction. Hence their concentration is taken as 1 to exclude them from equilibrium constant expression)
(concentration of pure solids remain constant during reaction. Hence their concentration is taken as 1 to exclude them from equilibrium constant expression)![K_{c}=\frac{[Ag^{+}]^{2}[CO_{3}^{2-}]}{[Ag^{+}]^{2}[CrO_{4}^{2-}]}](/tpl/images/0413/8617/3fc1b.png)