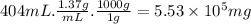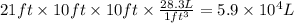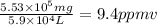Chemistry, 11.12.2019 18:31 serenityarts123
Aclumsy chemist drops a beaker containing 404 ml of a solvent (mw = 88.2, sg = 1.37) in a room measuring 21 ft x 10 ft x 10 ft. assuming that the contents of the beaker completely evaporate and fill the space, what is the resulting concentration in parts per million by volume (ppmv)? assume normal temperature and pressure (ntp), i. e., p = 1 atm, and t = 25 celsius. 1 ft3 = 28.3 l

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

You know the right answer?
Aclumsy chemist drops a beaker containing 404 ml of a solvent (mw = 88.2, sg = 1.37) in a room measu...
Questions



Mathematics, 25.08.2019 01:50



Geography, 25.08.2019 01:50

Computers and Technology, 25.08.2019 01:50

Biology, 25.08.2019 01:50

Social Studies, 25.08.2019 01:50

Biology, 25.08.2019 01:50



Social Studies, 25.08.2019 01:50

Mathematics, 25.08.2019 01:50

History, 25.08.2019 01:50



Health, 25.08.2019 01:50

Social Studies, 25.08.2019 01:50