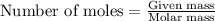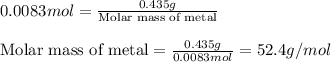A0.435 g sample of a metal, m, reacts completely with sulfuric acid according to m ( s ) + h 2 so 4 ( aq ) ⟶ mso 4 ( aq ) + h 2 ( g ) a volume of 201 ml of hydrogen is collected over water; the water level in the collecting vessel is the same as the outside level. atmospheric pressure is 756.0 torr, and the temperature is 25 °c. calculate the molar mass of the metal.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3
You know the right answer?
A0.435 g sample of a metal, m, reacts completely with sulfuric acid according to m ( s ) + h 2 so 4...
Questions




History, 21.09.2019 04:30


History, 21.09.2019 04:30



Mathematics, 21.09.2019 04:30




Social Studies, 21.09.2019 04:30

Chemistry, 21.09.2019 04:30




Health, 21.09.2019 04:30

Biology, 21.09.2019 04:30

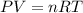
![25^oC=[25+273]K=298K](/tpl/images/0413/1404/df1f6.png)
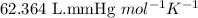

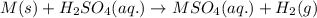
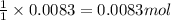 of metal
of metal