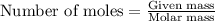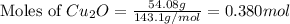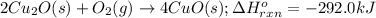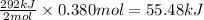Chemistry, 11.12.2019 05:31 Lydiac8715
The oxidation of copper(i) oxide, cu 2 o ( s ) , to copper(ii) oxide, cuo ( s ) , is an exothermic process. 2 cu 2 o ( s ) + o 2 ( g ) ⟶ 4 cuo ( s ) δ h ∘ rxn = − 292.0 kj mol calculate the energy released as heat when 54.08 g cu 2 o ( s ) undergo oxidation at constant pressure.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
The oxidation of copper(i) oxide, cu 2 o ( s ) , to copper(ii) oxide, cuo ( s ) , is an exothermic p...
Questions

English, 15.09.2020 21:01

English, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Mathematics, 15.09.2020 22:01

Physics, 15.09.2020 22:01

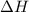 for the reaction will be -55.48 kJ
for the reaction will be -55.48 kJ