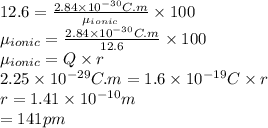Chemistry, 11.12.2019 04:31 4Myboyz1234
The dipole moment (μ) of hbr (a polar covalent molecule) is 0.851d (debye), and its percent ionic character is 12.6 % . estimate the bond length of the h−br bond in picometers. note that 1 d=3.34×10−30 c⋅m and

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

You know the right answer?
The dipole moment (μ) of hbr (a polar covalent molecule) is 0.851d (debye), and its percent ionic ch...
Questions


Chemistry, 04.03.2020 07:17


Mathematics, 04.03.2020 07:17

Mathematics, 04.03.2020 07:17

Chemistry, 04.03.2020 07:17

Mathematics, 04.03.2020 07:17


Mathematics, 04.03.2020 07:17

Mathematics, 04.03.2020 07:17

Mathematics, 04.03.2020 07:17



History, 04.03.2020 07:17


Social Studies, 04.03.2020 07:18

Mathematics, 04.03.2020 07:24


Mathematics, 04.03.2020 07:24

English, 04.03.2020 07:24

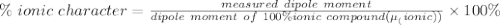
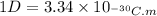
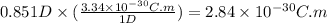
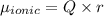
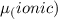 as follows:
as follows: