
Consider four different samples: aqueous lif, molten lif, aqueous agf, and molten agf. current run through each sample produces one of the following products at the cathode: solid lithium, solid silver, or hydrogen gas. match each sample to its cathodic product. drag the appropriate items to their respective bins.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Pbco3 –> pbo+ co2. how many liters of carbon dioxide gas is produced from the decomposition of 32 grams of lead (ll) carbonate?
Answers: 1

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
You know the right answer?
Consider four different samples: aqueous lif, molten lif, aqueous agf, and molten agf. current run...
Questions


Spanish, 21.01.2021 20:30

Chemistry, 21.01.2021 20:30


History, 21.01.2021 20:30



Mathematics, 21.01.2021 20:30


Mathematics, 21.01.2021 20:30


Chemistry, 21.01.2021 20:30

Mathematics, 21.01.2021 20:30

History, 21.01.2021 20:30


Chemistry, 21.01.2021 20:30




English, 21.01.2021 20:30

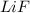 has water molecule, there will be competing will be two competing cations for reduction, while
has water molecule, there will be competing will be two competing cations for reduction, while 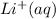 and
and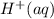 .However the reduction potential of
.However the reduction potential of 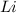 solid.
solid.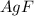 contains water molecules.there will be competing will be two competing cations for reduction, while
contains water molecules.there will be competing will be two competing cations for reduction, while 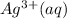 and
and

