
Chemistry, 10.12.2019 21:31 keasiabradley
Water's unique properties, including its high heat capacity, high density, high boiling point and a solid phase that is less dense than its liquid phase, can best be attributed to:
a) its formula, h2o.
b) the covalent oxygen-hydrogen bonds in the molecule.
c) the shape of the molecule.
d) the polarity of the molecule and hydrogen bonding between the molecules.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
Water's unique properties, including its high heat capacity, high density, high boiling point and a...
Questions




Mathematics, 05.05.2020 22:38


History, 05.05.2020 22:38

Biology, 05.05.2020 22:38

History, 05.05.2020 22:38

English, 05.05.2020 22:38

Medicine, 05.05.2020 22:38




Mathematics, 05.05.2020 22:39

Mathematics, 05.05.2020 22:39





Biology, 05.05.2020 22:39

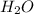 and due to the difference in electronegativity of hydrogen and oxygen atom there is formation of partial positive charge on hydrogen and partial negative charge on oxygen atom.
and due to the difference in electronegativity of hydrogen and oxygen atom there is formation of partial positive charge on hydrogen and partial negative charge on oxygen atom.

