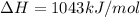Calculate the enthalpy of reaction for 2co + o2 → 2co2. given the following bond energies:
b...

Chemistry, 10.12.2019 19:31 cdjeter12oxoait
Calculate the enthalpy of reaction for 2co + o2 → 2co2. given the following bond energies:
be(c≡o) = 1074 kj/mol
be(o≡o) = 499 kj/mol
be(c≡o) = 802 kj/mol

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1
You know the right answer?
Questions



Mathematics, 15.12.2020 21:40

Chemistry, 15.12.2020 21:40

Chemistry, 15.12.2020 21:40


Mathematics, 15.12.2020 21:40

Mathematics, 15.12.2020 21:40

Mathematics, 15.12.2020 21:40


History, 15.12.2020 21:40


Mathematics, 15.12.2020 21:40


Mathematics, 15.12.2020 21:40



Mathematics, 15.12.2020 21:40


Mathematics, 15.12.2020 21:40

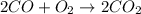
![\Delta H=[(2\times B.E_{C\equiv O})+(1\times B.E_{O\equiv O})]-[2\times B.E_{C=O}]](/tpl/images/0412/0179/8905d.png)
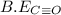 = 1074 kJ/mol
= 1074 kJ/mol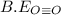 = 499 kJ/mol
= 499 kJ/mol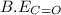 = 802 kJ/mol
= 802 kJ/mol![\Delta H=[(2\times 1074kJ/mol)+(1\times 499kJ/mol)]-[2\times 802kJ/mol]](/tpl/images/0412/0179/c40d4.png)