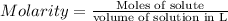Chemistry, 10.12.2019 09:31 flower1750
A3-m solution of vinegar (acetic acid and water) and a 3-m solution of salt water are both prepared. which has the greater molarity?
salt water and vinegar have equal molarities.
salt water will have the greater molarity because its molar mass is higher.
vinegar will have the greater molarity because its molar mass is higher.
it is impossible to determine with the information given.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1
You know the right answer?
A3-m solution of vinegar (acetic acid and water) and a 3-m solution of salt water are both prepared....
Questions

Biology, 23.06.2019 05:30


Mathematics, 23.06.2019 05:30


Biology, 23.06.2019 05:30


Biology, 23.06.2019 05:30

Mathematics, 23.06.2019 05:30

Mathematics, 23.06.2019 05:30

History, 23.06.2019 05:30




Mathematics, 23.06.2019 05:30

Advanced Placement (AP), 23.06.2019 05:30