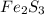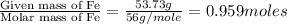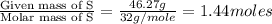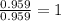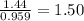Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Does anyone know a lot about how to: - calculate mass of magnesium metal - calculate the actual yield of magnesium oxide - calculate the theoretical yield of mgo - calculate the percent yield of mgo - determine the percent yield of mgo - determine the average percent yield of mgo i had to do an online lab and its asking these questions but i have no idea where to start or how to be able to find these things. i can post the chart of the data from the lab or if you can tell me exactly how i can find each.
Answers: 3


Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1
You know the right answer?
What is the empirical formula for a compound which contains 53.73% fe and 46.27% s...
Questions



Health, 04.07.2019 16:30


Mathematics, 04.07.2019 16:30

Mathematics, 04.07.2019 16:30


History, 04.07.2019 16:30


Mathematics, 04.07.2019 16:30


Mathematics, 04.07.2019 16:30

Mathematics, 04.07.2019 16:30

History, 04.07.2019 16:30

Mathematics, 04.07.2019 16:30



Biology, 04.07.2019 16:30

Mathematics, 04.07.2019 16:30