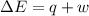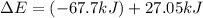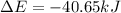Chemistry, 10.12.2019 06:31 wendelkristen
Consider a balloon filled with helium at the following conditions. 313 g he 1.00 atm 1910. l molar heat capacity = 20.8 j/degree c middot mol the temperature of this balloon is decreased by 41.6 degree c as the volume decreases to 1643 l with the pressure remaining constant. determine q, w, and delta e (in kj) for the compression of the balloon.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1
You know the right answer?
Consider a balloon filled with helium at the following conditions. 313 g he 1.00 atm 1910. l molar h...
Questions

English, 12.03.2021 19:10

Mathematics, 12.03.2021 19:10

Physics, 12.03.2021 19:10

Mathematics, 12.03.2021 19:10


Mathematics, 12.03.2021 19:10

Mathematics, 12.03.2021 19:10

Mathematics, 12.03.2021 19:10


Spanish, 12.03.2021 19:10


Mathematics, 12.03.2021 19:10

Mathematics, 12.03.2021 19:10

English, 12.03.2021 19:10




Mathematics, 12.03.2021 19:10


Mathematics, 12.03.2021 19:10

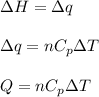
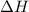 = change in enthalpy energy
= change in enthalpy energy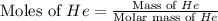
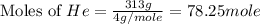
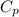 = heat capacity at constant pressure =
= heat capacity at constant pressure = 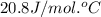
 = change in temperature =
= change in temperature = 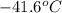
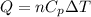
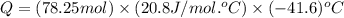
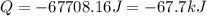
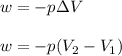
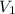 = initial volume = 1910 L
= initial volume = 1910 L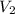 = final volume = 1643 L
= final volume = 1643 L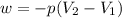
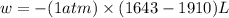
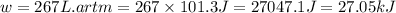
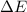 of the gas.
of the gas.