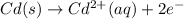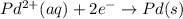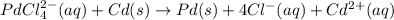Chemistry, 10.12.2019 06:31 antoinewill05
Avoltaic cell that uses the reaction pdcl2−4(aq)+cd(s)→pd(s)+4cl−(aq)+cd 2+(aq) has a measured standard cell potential of +1.03 v.
part a) write the two half-cell reactions. express your answer as a chemical equation. identify all of the phases in your answer.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2


Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
Avoltaic cell that uses the reaction pdcl2−4(aq)+cd(s)→pd(s)+4cl−(aq)+cd 2+(aq) has a measured stand...
Questions









Mathematics, 16.10.2020 07:01



Chemistry, 16.10.2020 07:01


Engineering, 16.10.2020 07:01


Mathematics, 16.10.2020 07:01


History, 16.10.2020 07:01

Physics, 16.10.2020 07:01