

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

You know the right answer?
Suppose you have a 0.10 m solution of albr3. what is the concentration of each ion? a. 0.10 m al3+...
Questions

Chemistry, 27.08.2019 00:30



English, 27.08.2019 00:30








Mathematics, 27.08.2019 00:30

Mathematics, 27.08.2019 00:30


Mathematics, 27.08.2019 00:30





Health, 27.08.2019 00:30

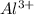 ions is 0.10 M and that of
ions is 0.10 M and that of 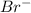 ions is 0.30 M
ions is 0.30 M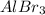 = 0.10 M
= 0.10 M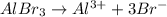
![[(3\times 0.1)]=0.3M](/tpl/images/0411/3441/9d959.png)


