
Chemistry, 10.12.2019 04:31 monsterwins5001
Ammonia will decompose into nitrogen and hydrogen at high temperature. an industrial chemist studying this reaction fills a 500. ml flask with 2.3 atm of ammonia gas at 32. °c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of hydrogen gas to be 0.69 atm. calculate the pressure equilibrium constant for the decomposition of ammonia at the final temperature of the mixture. round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
Ammonia will decompose into nitrogen and hydrogen at high temperature. an industrial chemist studyin...
Questions

Mathematics, 11.06.2020 17:57





Mathematics, 11.06.2020 17:57



History, 11.06.2020 17:57



Mathematics, 11.06.2020 17:57




Chemistry, 11.06.2020 17:57

History, 11.06.2020 17:57


Mathematics, 11.06.2020 17:57

Law, 11.06.2020 17:57

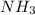 is as follows:.
is as follows:.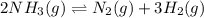
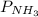 = 2.3 atm at equilibrium
= 2.3 atm at equilibrium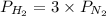 = 0.69 atm
= 0.69 atm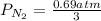
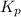 will be as follows.
will be as follows.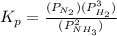
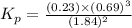
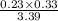
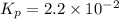
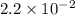 .
.

