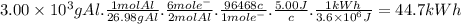The most useful ore of aluminum is bauxite, in which al is present as hydrated oxides, al2o3 * xh2o.
a) the number of kilowatt-hours of electricity required to produce 3.00kg of aluminum from electrolysis of compounds from bauxite is when the applied emf is 5.00 v.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
The most useful ore of aluminum is bauxite, in which al is present as hydrated oxides, al2o3 * xh2o....
Questions

Geography, 26.03.2021 04:00

Biology, 26.03.2021 04:00

Mathematics, 26.03.2021 04:00

Advanced Placement (AP), 26.03.2021 04:00

Mathematics, 26.03.2021 04:00

Mathematics, 26.03.2021 04:00

Mathematics, 26.03.2021 04:00

Mathematics, 26.03.2021 04:00


Mathematics, 26.03.2021 04:00




History, 26.03.2021 04:10

Mathematics, 26.03.2021 04:10

Mathematics, 26.03.2021 04:10

History, 26.03.2021 04:10


Mathematics, 26.03.2021 04:10

Mathematics, 26.03.2021 04:10