
Chemistry, 10.12.2019 04:31 sbudlove2838
Gfind the ∆hrxn for the reaction: 3c(s)+4h2(g) →c3h8(g) using these reactions with known ∆h’s: c3h8(g) + 5o2(g) → 3co2(g) + 4h2o(g) ∆h = −2043 kj c(s) + o2(g) → co2(g) ∆h = −393.5 kj 2h2(g) + o2(g) → 2h2o(g) ∆h = −483.6 kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1


Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1
You know the right answer?
Gfind the ∆hrxn for the reaction: 3c(s)+4h2(g) →c3h8(g) using these reactions with known ∆h’s: c3h...
Questions



Biology, 19.10.2021 02:30


Mathematics, 19.10.2021 02:30

Mathematics, 19.10.2021 02:30

Mathematics, 19.10.2021 02:30

English, 19.10.2021 02:30


Mathematics, 19.10.2021 02:30

English, 19.10.2021 02:30

Mathematics, 19.10.2021 02:30


Health, 19.10.2021 02:30



Geography, 19.10.2021 02:30


History, 19.10.2021 02:30

Mathematics, 19.10.2021 02:30

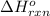 for the reaction is -104.7 kJ.
for the reaction is -104.7 kJ.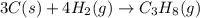
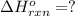
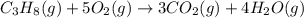
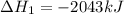
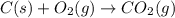
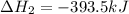 ( × 3)
( × 3) 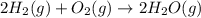
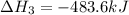 ( × 2)
( × 2) ![\Delta H^o_{rxn}=[1\times (-\Delta H_1)]+[3\times \Delta H_2]+[2\times \Delta H_3]](/tpl/images/0411/2485/b4dbe.png)
![\Delta H^o_{rxn}=[(1\times (-(-2043))+(3\times (-393.5))+(2\times (-483.6))]=-104.7kJ](/tpl/images/0411/2485/eafbb.png)


