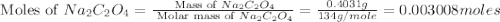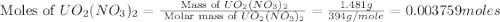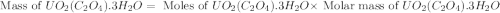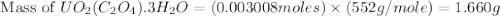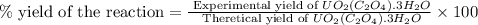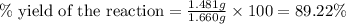Chemistry, 10.12.2019 04:31 hemolelekeakua
Uranium can be isolated from its ores by dissolving it as uo2(no3)2, then separating it as solid uo2(c2o4)*3h2o. addition of 0.4031 g of sodium oxalate, na2c2o4, to a solution containing 1.481 g of uranyl nitrate, uo2(no3)2, yields 1.073 g of solid uo2(c2o4)*3h2o.
na2c2o4 + uo2(no3)2 + 3h2o > uo2(c2o4)*3h2o + 2nano3
1. determine the limiting reactant and the percent yield of this reaction?

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3
You know the right answer?
Uranium can be isolated from its ores by dissolving it as uo2(no3)2, then separating it as solid uo2...
Questions

Arts, 24.04.2020 19:49

English, 24.04.2020 19:49

Mathematics, 24.04.2020 19:49

Biology, 24.04.2020 19:49






Mathematics, 24.04.2020 19:49

Biology, 24.04.2020 19:49


Mathematics, 24.04.2020 19:49

Social Studies, 24.04.2020 19:49


Computers and Technology, 24.04.2020 19:49



Computers and Technology, 24.04.2020 19:49

History, 24.04.2020 19:49


 = 1.481 g
= 1.481 g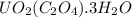 = 552 g/mole
= 552 g/mole