
Consider two flasks at 25 degree celsius, one contains an ideal gas and the other contains the real gas so3. which statement regarding these gases is true?
a. as the temperature approaches 0 k, the volume of the ideal gas will be larger than the volume of so3 because ideal gases lack inter-molecular forces.
b. as the temperature is decreased, the ideal gas will liquefy first because ideal gases have larger values of the van der waals coefficient b.
c. as the temperature approaches 0 k, the volume of the ideal gas will be smaller than the volume of so3 because ideal gases have larger values of the van der waals coefficient a.
d. after the temperature has been lowered about 100 k, the pressure of the ideal gas will be smaller than the pressure of so3 because the van der waals coefficient a for so3 is large.
e. after the temperature is increased about 100 k, the pressure of the ideal gas will be smaller than the pressure of so3 because the van der waals coefficient a for so3 is lar

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

You know the right answer?
Consider two flasks at 25 degree celsius, one contains an ideal gas and the other contains the real...
Questions

Mathematics, 23.05.2020 22:03

Mathematics, 23.05.2020 22:03


Social Studies, 23.05.2020 22:03





Chemistry, 23.05.2020 22:03


Engineering, 23.05.2020 22:03



Mathematics, 23.05.2020 22:03

Mathematics, 23.05.2020 22:03

Biology, 23.05.2020 22:03


Mathematics, 23.05.2020 22:03

Mathematics, 23.05.2020 22:03

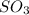 because ideal gases lack inter-molecular forces, is true.
because ideal gases lack inter-molecular forces, is true.

