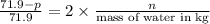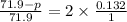Asolution of sodium chloride in water has a vapor pressure of 17.5 torr at 25°c. what is the mole fraction of nacl solute particles in this solution? what would be the vapor pressure of this solution at 45°c? the vapor pressure of pure water is 23.8 torr at 25°c and 71.9 torr at 45°c and assume sodium chloride exists as na⁺ and cl⁻ ions in solution.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1


Chemistry, 23.06.2019 07:40
Did the detergents containing enzymes work better at removing stains than those containing no enzyme? why or why not?
Answers: 2
You know the right answer?
Asolution of sodium chloride in water has a vapor pressure of 17.5 torr at 25°c. what is the mole fr...
Questions

Mathematics, 03.09.2021 21:00

Chemistry, 03.09.2021 21:00




Mathematics, 03.09.2021 21:00

Mathematics, 03.09.2021 21:00




English, 03.09.2021 21:00




Arts, 03.09.2021 21:00


Social Studies, 03.09.2021 21:00

Mathematics, 03.09.2021 21:00


Mathematics, 03.09.2021 21:00

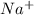 and
and 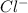 in solution. Therefore, Van't Hoff factor (i) will be equal to 2.
in solution. Therefore, Van't Hoff factor (i) will be equal to 2.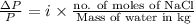
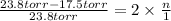
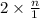
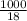
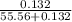
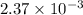
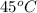 , the vapor pressure will be calculated as follows.
, the vapor pressure will be calculated as follows.