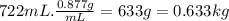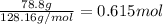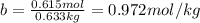Chemistry, 10.12.2019 01:31 erinwebsterrr
Determine the freezing point of a solution that contains 78.8 g of naphthalene (c10h8, molar mass = 128.16 g/mol) dissolved in 722 ml of benzene (d = 0.877 g/ml). pure benzene has a melting point of 5.50°c and a freezing point depression constant of 4.90°c/m.0.74°c4.76°c4.17°c1.68°c1. 33°c

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
Determine the freezing point of a solution that contains 78.8 g of naphthalene (c10h8, molar mass =...
Questions


History, 07.03.2020 01:03


Mathematics, 07.03.2020 01:03









Computers and Technology, 07.03.2020 01:03



Computers and Technology, 07.03.2020 01:03

Mathematics, 07.03.2020 01:03

Business, 07.03.2020 01:03


Mathematics, 07.03.2020 01:03