
Chemistry, 09.12.2019 21:31 Knownothing
Fe(ii) can be precipitated from a slightly basic aqueous solution by bubbling oxygen through the solution, which converts fe(ii) to insoluble fe(iii):
4fe(oh)+(aq)+ 4oh-(aq)+o2(g)+2h2o(l) = 4fe(oh)3(s)
how many grams of o2 are consumed to precipitate all of the iron in 85ml of 0.075 m fe(ii).
explain step by step

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Which of these will change if the air in aclosed bottle is heated? abcdthe mass of the airthe composition of the airthe air pressure in the bottlethe number of air molecules in the bottle
Answers: 3



Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1
You know the right answer?
Fe(ii) can be precipitated from a slightly basic aqueous solution by bubbling oxygen through the sol...
Questions


Mathematics, 23.10.2020 03:01


Computers and Technology, 23.10.2020 03:01

Mathematics, 23.10.2020 03:01


Mathematics, 23.10.2020 03:01

Mathematics, 23.10.2020 03:01



History, 23.10.2020 03:01

Mathematics, 23.10.2020 03:01


History, 23.10.2020 03:01

Mathematics, 23.10.2020 03:01

History, 23.10.2020 03:01


Business, 23.10.2020 03:01

Chemistry, 23.10.2020 03:01

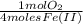 =
= 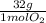 = 0,051g of O₂
= 0,051g of O₂

