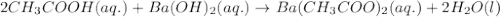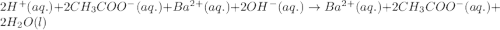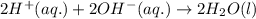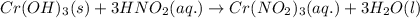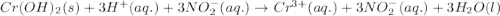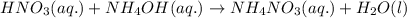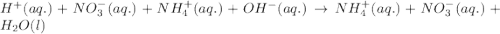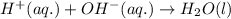Chemistry, 23.11.2019 09:31 sepdentalcare8774
Write the balanced molecular and net ionic equations for each of the following neutralization reactions. (a) aqueous acetic acid (hc2h3o2) is neutralized by aqueous barium hydroxide. (b) solid chromium(iii) hydroxide reacts with nitrous acid. (c) aqueous nitric acid and aqueous ammonia react.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

You know the right answer?
Write the balanced molecular and net ionic equations for each of the following neutralization reacti...
Questions

Geography, 21.07.2021 04:10





English, 21.07.2021 04:10



Mathematics, 21.07.2021 04:10



Mathematics, 21.07.2021 04:10


Business, 21.07.2021 04:10

Mathematics, 21.07.2021 04:10

Mathematics, 21.07.2021 04:10



Mathematics, 21.07.2021 04:10

Mathematics, 21.07.2021 04:10

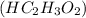 is neutralized by aqueous barium hydroxide.
is neutralized by aqueous barium hydroxide.