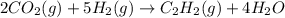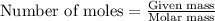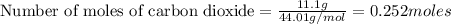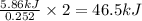Chemistry, 09.12.2019 20:31 poptropic9207
The reaction of carbon dioxide(g) with hydrogen(g) to form acetylene(g) (c2h2) and water(g) proceeds as follows: 2 co2(g) + 5 h2(g) c2h2(g) + 4 h2o(g) when 11.1 grams of co2(g) react with sufficient h2(g) , 5.86 kj of energy are absorbed . what is the value of h for the chemical equation given? δhrxn = kj

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:40
What type of solution is formed if 10 g of kclo3 are dissolved in 100g of water at 30
Answers: 2

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
The reaction of carbon dioxide(g) with hydrogen(g) to form acetylene(g) (c2h2) and water(g) proceeds...
Questions

Advanced Placement (AP), 08.11.2020 09:30


Mathematics, 08.11.2020 09:30

Mathematics, 08.11.2020 09:30

Social Studies, 08.11.2020 09:30



Social Studies, 08.11.2020 09:30




Mathematics, 08.11.2020 09:30

Mathematics, 08.11.2020 09:30

Chemistry, 08.11.2020 09:30

Social Studies, 08.11.2020 09:30

English, 08.11.2020 09:30