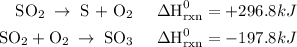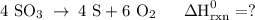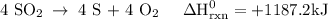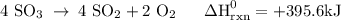Chemistry, 09.12.2019 18:31 ahhhhhhhh5509
Use the standard reaction enthalpies given below to determine δh°rxn for the following reaction: 4 so3(g) → 4 s(s) + 6 o2(g) δh°rxn = ? given: so2(g) → s(s) + o2(g) δh°rxn = +296.8 kj 2 so2(g) + o2(g) → 2 so3(g) δh°rxn = -197.8 kj

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:50
Your roll: experienced electron speech is adressed to: a new "freshman class" of electrons job: write a speech task: you are to pretend that you are giving a speech to a new group of electrons. be sure to mention their placement in an atom, their charge, and their role in chemical bonding (ionic and covalent) be specific!
Answers: 3

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Use the standard reaction enthalpies given below to determine δh°rxn for the following reaction: 4...
Questions


History, 26.09.2019 21:40


Mathematics, 26.09.2019 21:40

Mathematics, 26.09.2019 21:40

Mathematics, 26.09.2019 21:40

Mathematics, 26.09.2019 21:40

History, 26.09.2019 21:40




Physics, 26.09.2019 21:40

Mathematics, 26.09.2019 21:40


Mathematics, 26.09.2019 21:40


Mathematics, 26.09.2019 21:40


Computers and Technology, 26.09.2019 21:40

Social Studies, 26.09.2019 21:40

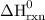 = 1582.8 kJ.
= 1582.8 kJ.