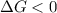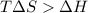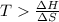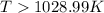Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1
You know the right answer?
For the reaction c(s)+h2o(g)→co2(g)+h2(g) δh∘=131.3kj/mol and δs∘=127.6j/k⋅mol at 298k. at temperatu...
Questions


Mathematics, 04.11.2019 22:31

Mathematics, 04.11.2019 22:31


Mathematics, 04.11.2019 22:31

History, 04.11.2019 22:31




Mathematics, 04.11.2019 22:31


History, 04.11.2019 22:31

Mathematics, 04.11.2019 22:31

History, 04.11.2019 22:31

Mathematics, 04.11.2019 22:31

Geography, 04.11.2019 22:31

English, 04.11.2019 22:31

English, 04.11.2019 22:31

Physics, 04.11.2019 22:31

 this reaction is spontaneous under standard conditions.
this reaction is spontaneous under standard conditions.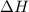 = 131.3 KJ/mole = 131300 J/mole
= 131.3 KJ/mole = 131300 J/mole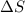 = 127.6 J/mole.K
= 127.6 J/mole.K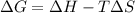
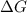 is negative or we can say that the value of
is negative or we can say that the value of