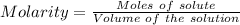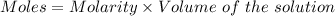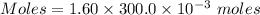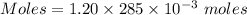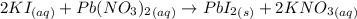You mix 285.0 ml of 1.20 m lead(ii) nitrate with 300.0 ml of 1.60 m potassium iodide. the lead(ii) iodide is insoluble. which of the following is false?
select one:
a. the final concentration of pb2+ ions is 0.174 m.
b. you form 111 g of lead(ii) iodide.
c. the final concentration of k+ is 0.821 m.
d. the final concentration of no3– is 0.821 m.
e. all are true.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 23.06.2019 02:30
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2
You know the right answer?
You mix 285.0 ml of 1.20 m lead(ii) nitrate with 300.0 ml of 1.60 m potassium iodide. the lead(ii) i...
Questions

Physics, 06.07.2020 16:01


Mathematics, 06.07.2020 16:01

Mathematics, 06.07.2020 16:01

Mathematics, 06.07.2020 16:01

History, 06.07.2020 16:01


Mathematics, 06.07.2020 16:01



Mathematics, 06.07.2020 16:01

Mathematics, 06.07.2020 16:01

English, 06.07.2020 17:01




Physics, 06.07.2020 17:01

Biology, 06.07.2020 17:01

Mathematics, 06.07.2020 17:01

Mathematics, 06.07.2020 17:01