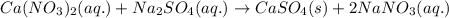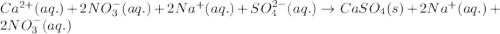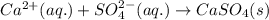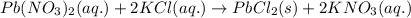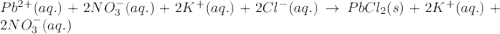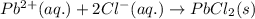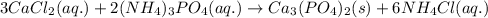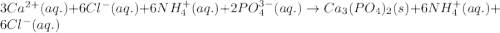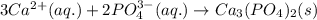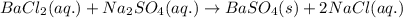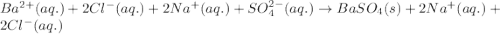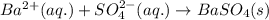Chemistry, 09.12.2019 17:31 jbehrens6538
Write the net ionic equation to show the formation of a solid (insoluble salt) when the following solutions are mixed. write noreaction if there is no precipitate. express your answer as a chemical equation. identify all of the phases in your answer. enter noreaction if no precipitate is formed.1. ca(no3)2(aq) + na2so4(aq)2. kcl(aq) + pb(no3)2(aq)3. cacl2(aq) + (nh4)3(po4)(aq)4. na2so4(aq) + bacl2(aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1
You know the right answer?
Write the net ionic equation to show the formation of a solid (insoluble salt) when the following so...
Questions

History, 17.06.2021 06:30


Mathematics, 17.06.2021 06:30

Mathematics, 17.06.2021 06:30


Social Studies, 17.06.2021 06:30

Chemistry, 17.06.2021 06:30


Social Studies, 17.06.2021 06:30


Mathematics, 17.06.2021 06:30

Mathematics, 17.06.2021 06:30


History, 17.06.2021 06:30

Mathematics, 17.06.2021 06:30


Mathematics, 17.06.2021 06:30



English, 17.06.2021 06:30