
Chemistry, 09.12.2019 17:31 makayla4141
X2 + y2 > x2y2 rate = k[x2]a reaction and its experimentally determined rate law are represented above. a chemist proposes two different possible mechanisms for the reaction, which are given below. mechanism 1 mechanism 2x2 > 2x (slow) x2 > 2x (slow)x + y2 > xy2 (fast) x + y2 > xy + y (fast)x + xy2 > x2y2 (fast) x + xy > x2y (fast)x2y + y > x2y2 (fast)based on the information above, which of the following is true? both mechanism 1 and 2 are consistent with the rate law. only mechanism 2 is consistent with the rate law. neither mechanism 1 nor mechanism 2 is consistent with the rate law. only mechanism 1 is consistent with the rate law.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1
You know the right answer?
X2 + y2 > x2y2 rate = k[x2]a reaction and its experimentally determined rate law are represented...
Questions


Mathematics, 25.01.2021 17:40


Social Studies, 25.01.2021 17:40

Mathematics, 25.01.2021 17:40


Mathematics, 25.01.2021 17:40

Mathematics, 25.01.2021 17:40


Arts, 25.01.2021 17:40



Social Studies, 25.01.2021 17:40



English, 25.01.2021 17:40

Mathematics, 25.01.2021 17:40

Mathematics, 25.01.2021 17:40

Mathematics, 25.01.2021 17:40

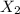 ⇒
⇒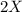 is the slowest step or RATE DETERMINING STEP .
is the slowest step or RATE DETERMINING STEP .![[X_2]](/tpl/images/0410/1202/df4e1.png) .
.



