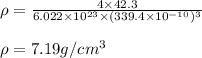Chemistry, 22.08.2019 08:30 domenica19
The atomic radius of metal x is 1.20 × 102 picometers (pm) and a crystal of metal x has a unit cell that is face-centered cubic. calculate the density of metal x? (atomic weight = 42.3 g/mol)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
The atomic radius of metal x is 1.20 × 102 picometers (pm) and a crystal of metal x has a unit cell...
Questions


Mathematics, 10.10.2019 08:00

Mathematics, 10.10.2019 08:00

Geography, 10.10.2019 08:00

Biology, 10.10.2019 08:00



Biology, 10.10.2019 08:00

Physics, 10.10.2019 08:00

Computers and Technology, 10.10.2019 08:00


English, 10.10.2019 08:00

English, 10.10.2019 08:00




Social Studies, 10.10.2019 08:00

Mathematics, 10.10.2019 08:00

Geography, 10.10.2019 08:00

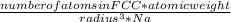 . Substituting the given, the density is 162.69 g/cm3.
. Substituting the given, the density is 162.69 g/cm3. 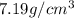
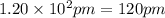
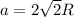
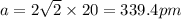
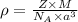
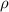 = density
= density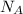 = Avogadro's number =
= Avogadro's number = 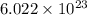
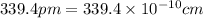 (Conversion factor:
(Conversion factor: 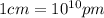 )
)