
Chemistry, 01.12.2019 00:31 deaishaajennings123
Calculate the approximate enthalpy change, δhrxn, for the combustion of methane: ch4+2o2→2h2o+co2 δhrxn from a given table: ch4 = 1656 kj/mol o2 = 498 kj/mol h2o = 928 kj/mol co2 = 1598 kj/mol?

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

You know the right answer?
Calculate the approximate enthalpy change, δhrxn, for the combustion of methane: ch4+2o2→2h2o+co2 δ...
Questions










Mathematics, 12.08.2020 05:01










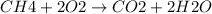
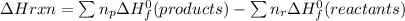
![\Delta Hrxn = [2\Delta H_{f}^{0}(H2O)+1\Delta H_{f}^{0}(CO2)]-[1\Delta H_{f}^{0}(CH4)+ 2\Delta H_{f}^{0}(O2)]](/tpl/images/0397/7957/b0337.png)



