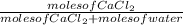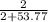Chemistry, 07.12.2019 06:31 pinapunapula
A2.00 m solution of cacl2 (111 g/mol) in water has a density of 1.17 g/ml. what is the mole fraction of cacl2? enter your answer with 4 decimal places. hint: start with 1.00 l of solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
A2.00 m solution of cacl2 (111 g/mol) in water has a density of 1.17 g/ml. what is the mole fraction...
Questions

Business, 13.01.2020 07:31

History, 13.01.2020 07:31



Mathematics, 13.01.2020 07:31

Mathematics, 13.01.2020 07:31

Social Studies, 13.01.2020 07:31

Computers and Technology, 13.01.2020 07:31







Mathematics, 13.01.2020 07:31

English, 13.01.2020 07:31

Biology, 13.01.2020 07:31

Mathematics, 13.01.2020 07:31

Mathematics, 13.01.2020 07:31

English, 13.01.2020 07:31