

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
An atom (not a hydrogen atom) absorbs a photon whose associated wavelength is 300 nm and then immedi...
Questions


Mathematics, 17.01.2020 13:31

Chemistry, 17.01.2020 13:31


English, 17.01.2020 13:31

History, 17.01.2020 13:31



Mathematics, 17.01.2020 13:31


History, 17.01.2020 13:31

Geography, 17.01.2020 13:31


World Languages, 17.01.2020 13:31

Mathematics, 17.01.2020 13:31

Mathematics, 17.01.2020 13:31

Mathematics, 17.01.2020 13:31

English, 17.01.2020 13:31

Spanish, 17.01.2020 13:31

 - 1/λ
- 1/λ )
)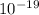 J.
J.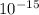 eVs
eVs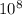 m/s
m/s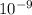 m
m )
) 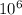 eV = 2.196 eV
eV = 2.196 eV


