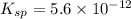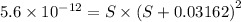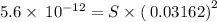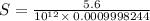Chemistry, 07.12.2019 05:31 NeverEndingCycle
What is the molar solubility of mg(oh)2 in a basic solution with a ph of 12.50? ksp for mg(oh)2 is 5.6 × 10-12. what is the molar solubility of mg(oh)2 in a basic solution with a ph of 12.50? ksp for mg(oh)2 is 5.6 × 10-12. 1.1 × 10-4 m 5.6 × 10-9 m 2.4 × 10-6 m 1.8 × 10-10 m

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
What is the molar solubility of mg(oh)2 in a basic solution with a ph of 12.50? ksp for mg(oh)2 is...
Questions

Spanish, 15.12.2020 06:20

Mathematics, 15.12.2020 06:20



Physics, 15.12.2020 06:20

Mathematics, 15.12.2020 06:20


Mathematics, 15.12.2020 06:20

Chemistry, 15.12.2020 06:20



Mathematics, 15.12.2020 06:20

Chemistry, 15.12.2020 06:20



Mathematics, 15.12.2020 06:20

Mathematics, 15.12.2020 06:20

English, 15.12.2020 06:20

Mathematics, 15.12.2020 06:20

History, 15.12.2020 06:20

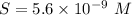
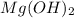 will form its respective ions in the solution as:
will form its respective ions in the solution as:
![K_{sp}=[Mg^{2+}][OH^-]^2](/tpl/images/0407/6768/48330.png)
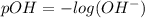
![[OH^-]=10^{(-1.5)}=0.03162](/tpl/images/0407/6768/a8c86.png)