
Chemistry, 07.12.2019 04:31 maxy7347go
In which solution(s) will a precipitate form? i. 0.00090 g of na2cro4 (molar mass = 162.0 g/mol) is added to 225 ml of 0.00025 m agno3. ksp ag2cro4 =1.1 x 10-12 ii. 0.100l of 0.0015 m mgcl2 and 0.200l of 0.012 m naf. ksp mgf2 = 3.7 x 10-8

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
In which solution(s) will a precipitate form? i. 0.00090 g of na2cro4 (molar mass = 162.0 g/mol) is...
Questions


Physics, 04.09.2019 19:30

English, 04.09.2019 19:30




Social Studies, 04.09.2019 19:30

Physics, 04.09.2019 19:30







Biology, 04.09.2019 19:30





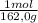 = 5,56x10⁻⁶ moles of Na₂CrO₄≡ moles of CrO₄²⁻.
= 5,56x10⁻⁶ moles of Na₂CrO₄≡ moles of CrO₄²⁻.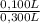 = 0,0005M MgCl₂≡ Mg²⁺
= 0,0005M MgCl₂≡ Mg²⁺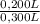 = 0,008M NaF ≡ F⁻
= 0,008M NaF ≡ F⁻

