
Chemistry, 07.12.2019 04:31 rleiphart1
A) moles of co2 required to inflate 160-l front passenger-side air bag at room temperature and pressure
b) balanced equation for the reaction of nahco3 and ch3cooh to co2
c)mass of nahco3 needed for the reaction (84.0 g/mol)
d) volume of vinegar required (0.833 m acetic acid)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1

Chemistry, 23.06.2019 05:30
According to thomson, the atom is a positively charged cloud with electrons scattered throughout. what would the alpha particles do when they hit the foil if thomson were correct
Answers: 1
You know the right answer?
A) moles of co2 required to inflate 160-l front passenger-side air bag at room temperature and press...
Questions



Mathematics, 05.03.2020 23:03





English, 05.03.2020 23:04






Mathematics, 05.03.2020 23:04





Mathematics, 05.03.2020 23:04

Mathematics, 05.03.2020 23:04

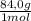 = 549g of NaHCO₃
= 549g of NaHCO₃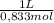 = 7,85L of CH₃COOH
= 7,85L of CH₃COOH

