Chemistry, 07.12.2019 03:31 rbeltran24
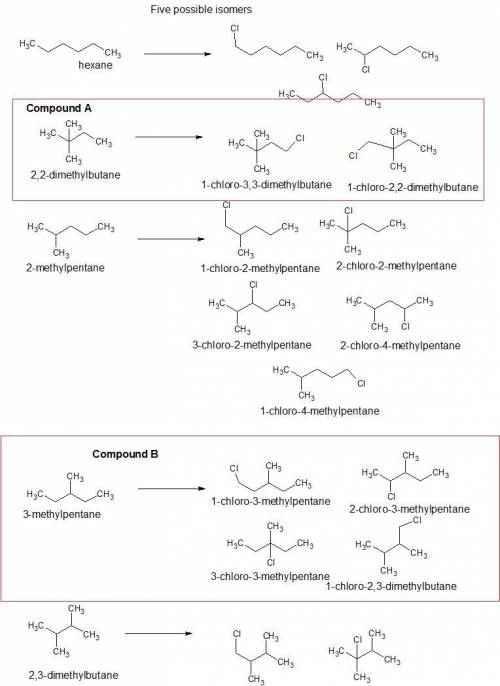
Both compounds a and b have molecular formula c6h14. monochlorination of compound a results in the formation of two constitutional isomers. monochlorination of compound b results in the formation of four constitutional isomers. identify compounds a and b, and show the products of each monochlorination.
1) draw compound a and the products of its monochlorination.
2)draw compound b and the products of its monochlorination.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1
You know the right answer?
Both compounds a and b have molecular formula c6h14. monochlorination of compound a results in the f...
Questions


Mathematics, 14.05.2020 12:57



English, 14.05.2020 12:57

Mathematics, 14.05.2020 12:57


Mathematics, 14.05.2020 12:57









History, 14.05.2020 12:57


Mathematics, 14.05.2020 12:57

Mathematics, 14.05.2020 12:57