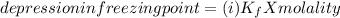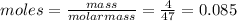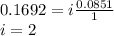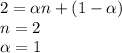Chemistry, 07.12.2019 02:31 kenzieeee96
When hno2 is dissolved in water it partially dissociates according to the equation hno2⇌h++no−2. a solution is prepared that contains 4.000 g of hno2 in 1.000 kg of water. its freezing point is found to be -0.1692 ∘c calculate the fraction of hno2 that has dissociated.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

You know the right answer?
When hno2 is dissolved in water it partially dissociates according to the equation hno2⇌h++no−2. a s...
Questions

History, 03.02.2022 08:00

Chemistry, 03.02.2022 08:00



SAT, 03.02.2022 08:10


Arts, 03.02.2022 08:10


SAT, 03.02.2022 08:10


Mathematics, 03.02.2022 08:10



Mathematics, 03.02.2022 08:10


English, 03.02.2022 08:10


Chemistry, 03.02.2022 08:10

Computers and Technology, 03.02.2022 08:10

Mathematics, 03.02.2022 08:10