Chemistry, 07.12.2019 01:31 pharadorvil04
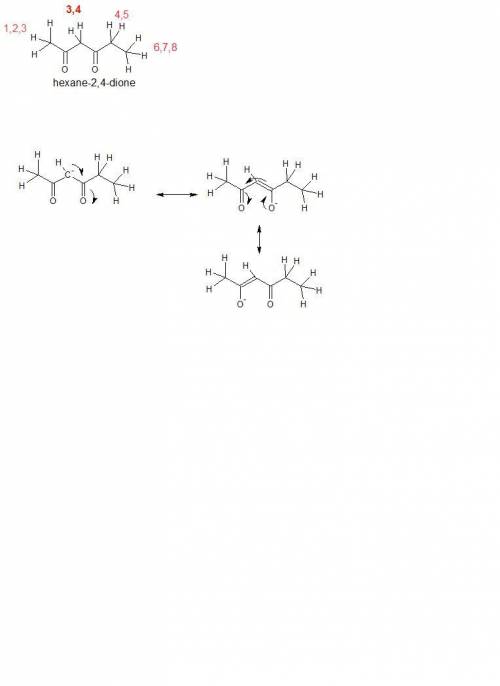
Using resonance, select the most acidic protons in the hexane-2,4-dione molecule. the most acidic proton is the one that, upon removal, will yield the most stable conjugate base.
check all that apply.
[hexane-2,4-dione molecule with protons numbered as follows according to iupac nomenclature: protons on c1 are numbered 1 through 3, protons on c3 are numbered 4 and 5, protons on c5 are numbered 6 and 7, protons on c6 are numbered 8 through 10.]
check all that apply.
protons 1, 2, or 3
protons 4 or 5
protons 6 or 7
protons 8, 9, or 10

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which mathematical relationship should you us to convert moles of a substance into grams
Answers: 1

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1


Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1
You know the right answer?
Using resonance, select the most acidic protons in the hexane-2,4-dione molecule. the most acidic pr...
Questions



Mathematics, 31.01.2020 23:51



Chemistry, 31.01.2020 23:51


Mathematics, 31.01.2020 23:51


Mathematics, 31.01.2020 23:51

Chemistry, 31.01.2020 23:51

Mathematics, 31.01.2020 23:51


Mathematics, 31.01.2020 23:51


History, 31.01.2020 23:51



Chemistry, 31.01.2020 23:51