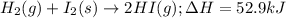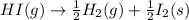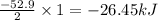Chemistry, 07.12.2019 00:31 hannahs1313
Given the following reaction: h2(g)+i2(s) → 2hi(g) with a ∆h of 52.9 kj. what is the change in enthalpy for the following reaction: hi(g) → 1 2h2(g)+1 2 i2(s)? express your answer in kj.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
Given the following reaction: h2(g)+i2(s) → 2hi(g) with a ∆h of 52.9 kj. what is the change in enth...
Questions