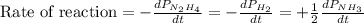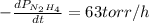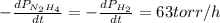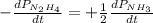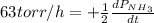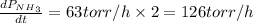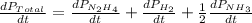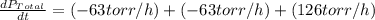Chemistry, 07.12.2019 00:31 kaylarenee05080
The rate of decrease in n2h4 partial pressure in a closed reaction vessel form the reaction: n2h4 (g) + h2 (g) \rightarrow 2 nh3 (g) is63 torr/h. what are the rates of change of nh3 partial pressure and total pressure in the vessel.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
The rate of decrease in n2h4 partial pressure in a closed reaction vessel form the reaction: n2h4 (...
Questions

History, 27.09.2019 19:40


Mathematics, 27.09.2019 19:40

History, 27.09.2019 19:40

Social Studies, 27.09.2019 19:40

Mathematics, 27.09.2019 19:40



Mathematics, 27.09.2019 19:40

History, 27.09.2019 19:40


Mathematics, 27.09.2019 19:40

Chemistry, 27.09.2019 19:40

Chemistry, 27.09.2019 19:40



Mathematics, 27.09.2019 19:40


History, 27.09.2019 19:40

English, 27.09.2019 19:40

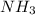 partial pressure is 126 torr/h.
partial pressure is 126 torr/h.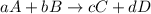
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0407/2122/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0407/2122/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0407/2122/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0407/2122/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0407/2122/d4b94.png)
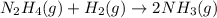
![\text{Rate of disappearance of }N_2H_4=-\frac{d[N_2H_4]}{dt}](/tpl/images/0407/2122/4b6d3.png)
![\text{Rate of disappearance of }H_2=-\frac{d[H_2]}{dt}](/tpl/images/0407/2122/53b46.png)
![\text{Rate of formation of }NH_3=+\frac{1}{2}\frac{d[NH_3]}{dt}](/tpl/images/0407/2122/f55ec.png)