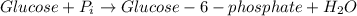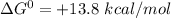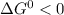Chemistry, 06.12.2019 23:31 deidaralove90
In the first step of glycolysis, the given two reactions are coupled. reaction 1: glucose + p i ⟶ glucose - 6 - phosphate + h 2 o δ g = + 13.8 k j / mol reaction 2: atp + h 2 o ⟶ adp + p i δ g = − 30.5 k j / mol answer the four questions about the first step of glycolysis. is reaction 1 spontaneous or nonspontaneous?

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2
You know the right answer?
In the first step of glycolysis, the given two reactions are coupled. reaction 1: glucose + p i ⟶ g...
Questions



Advanced Placement (AP), 16.10.2020 06:01




Health, 16.10.2020 06:01

Geography, 16.10.2020 06:01

English, 16.10.2020 06:01


Biology, 16.10.2020 06:01


Computers and Technology, 16.10.2020 06:01


Chemistry, 16.10.2020 06:01




Mathematics, 16.10.2020 06:01

Social Studies, 16.10.2020 06:01